-
Effectiveness of a mobile application for tracking symptoms and enhancing symptom management among breast cancer patients receiving chemotherapy in Bangkok, Thailand: a non-randomized controlled trial
-
Duangrat Kaveenuntachai, Supawan Jaiboon, Bualuang Sumdaengrit, Chureeporn Silaguntsuti, Arveewan Vittayatigonnasak, Pornchan Sailamai
-
J Korean Acad Nurs 2025;55(2):178-190. Published online May 27, 2025
-
DOI: https://doi.org/10.4040/jkan.25011
-
-
 Abstract Abstract
 PDF PDF ePub ePub
- Purpose
This study evaluated the effectiveness of a mobile application in tracking symptoms and improving symptom management and quality of life (QoL) among breast cancer patients undergoing chemotherapy in Thailand.
Methods
A non-randomized controlled trial was used, with 25 participants in the intervention group and 25 in the control group. Research instruments included a demographic data form, the NCI-PRO-CTCAE Items-Thai-Thailand version 1.0, and the European Organization for Research and Treatment of Cancer Quality of Life Core Questionnaire and Breast Cancer-Specific Module.
Results
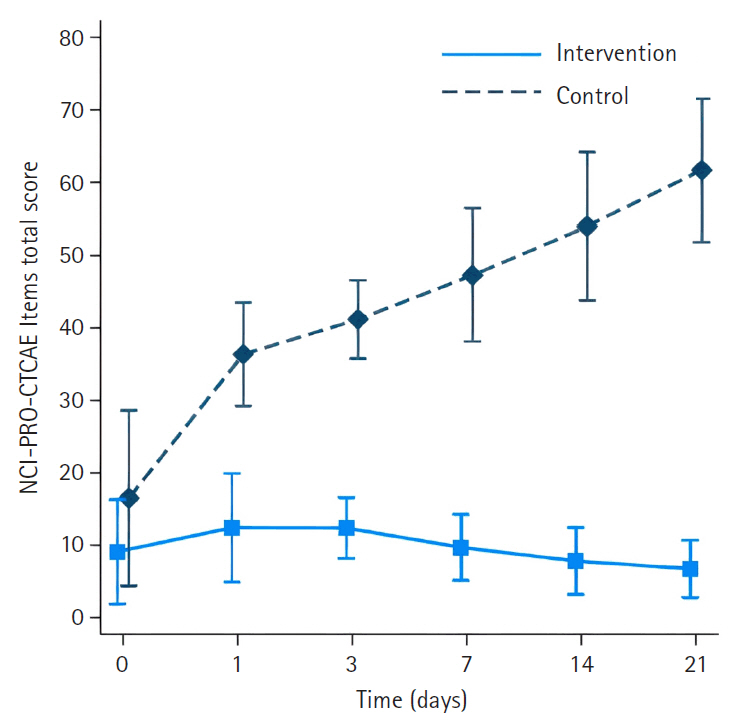
The intervention group had significantly less severe side effects than the control group, with mean differences of –23.33 (95% confidence interval [CI], –27.82 to –18.83) on day 1, –28.18 (95% CI, –33.22 to –23.14) on day 3, –34.63 (95% CI, –40.18 to –29.08) on day 7, –42.56 (95% CI, –48.72 to –36.40) on day 14, and –51.31 (95% CI, –58.13 to –44.48) on day 21 (p<.001 for all). On day 21, participants in the intervention group reported significantly higher scores in the Global Health QoL and Functional Scales compared to the control group (p<.001). Additionally, intervention group participants reported lower scores on the Symptom Scales and higher scores on the Functional Scales than those in the control group (p<.001).
Conclusion
The ChemoPro application helped manage chemotherapy-related symptoms and was associated with improved symptom monitoring and QoL. Nonetheless, the study was limited by a small sample size and restriction to Android users. Future research with larger and more diverse populations is recommended before broader implementation in clinical practice.
|